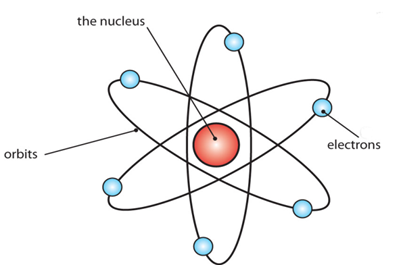
Atom is the smallest particle of a chemical element that can exist. An atom is made of up three types of particle called subatomic particles: Protons, neutrons and electrons. The protons and neutrons are making up the center of the atom called nucleus and the electron revolve around above the nucleus in small cloud pattern.
The existence of atom has been proposed by great Indian philosopher or sage Maharishi Kanad who discovered atom in 600BC and he named them Paramanus (‘param’ mean ultimate and ‘anu’ mean particle known as molecule). Since ancient times, these thinkers suspected that everything consisted of tiny particles. However, in the absence of any experimental proof, their atomic theories are solely based on logical and scientific thinking. In 400 BC Democritus was Greek philosophers who use the term atom (atomos; meaning indivisible).

He thought that if you take piece of matter and divide it and continue to divide it will eventually come to point where you could not divide it any more. This fundamental or basic unit was what Democritus called atom.

- He called this theory of the universe:
- All matter consists of atoms, which are bits of matter too small to be seen.
- There is an empty space between atoms.
- Atoms are completely solid.
- Atoms have no internal structure.
| Atoms are tiny particles of matterAtoms of an element similar and different from other element.Atoms of two or more different elements combine to form compounds. A given compound always has the same relative numbers and type of atoms.Atoms are rearranged to form new combinations in chemical reaction. |
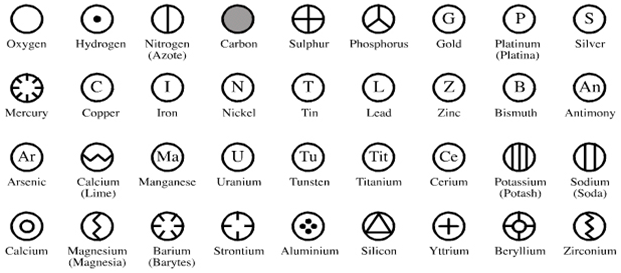
John Dalton (1766- 1844AD) was a science teacher who also kept detailed records of weather. He discovered that different element consisted of tiny particles, atoms. Dalton further discovered that atoms.
- Atoms are tiny particles of matter
- Atoms of an element similar and different from other element.
- Atoms of two or more different elements combine to form compounds. A given compound always has the same relative numbers and type of atoms.
- Atoms are rearranged to form new combinations in chemical reaction.

<