Current
It is simply the flow of electrons. A continuous flow of electrons or charged particles can be termed as Current. It is indicated by I or i. It is measured in Amperes. This can be alternating current AC or direct current DC.
Voltage
It is the potential difference. When there occurs a difference in potentialities, between two points, there is said to be a voltage difference, measured between those two points. It is indicated by V. It is measured in Volts.
Resistance
It is the property of opposing the flow of electrons. The possession of this property can be termed as resistivity. This will be discussed later in detail.
Ohm’s Law
With the terms discussed above, we have a standard law, which is very crucial for the behavior of all the electronic components, called as Ohm’s Law. This states the relation between current and voltage in an ideal conductor.
According to Ohm’s law, the potential difference across an ideal conductor is proportional to the current through it.
V α I
An ideal conductor has no resistance. But in practice, every conductor has some resistance in it. As the resistance increases, the potential drop also increases and hence the voltage increases.
Hence the voltage is directly proportional to the resistance it offers.
V α R
V=IR
But the current is inversely proportional to the resistance.
V α I α 1/R
I=V/R
Hence, in practice, an Ohm’s law can be stated as −
According to Ohm’s law, the current flowing through a conductor is proportional to the potential difference across it, and is inversely proportional to the resistance it offers.
This law is helpful in determining the values of unknown parameters among the three which help to analyze a circuit.
Fermi Level
This level refers to the highest occupied molecular orbital at absolute zero. It is usually found at the center between the valence and conduction bands. The particles in this state each have their own quantum states and generally do not interact with each other. When the temperature begins to rise above absolute zero, these particles will begin to occupy states above the Fermi level and states below the Fermi level become unoccupied.
Covalent bonding:
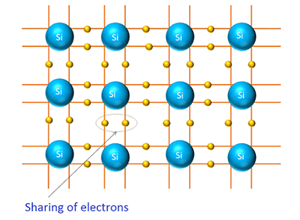
Covalent bonding in Si:
The outermost shell of atom is capable of hold to eight electrons. The atom which has eight electrons in the outermost orbit is said to be completely filled and most stable. But the outermost orbit of silicon has only four electrons, Si atom needs four more electrons to become more stable. Si atoms form four covalent bonds with four neighboring atoms. In covalent bonding each valance electrons is shared by two atoms.
When Si atoms comes close to each other, each valence electron of atoms is shared with neighboring atom and each valence electron of neighboring atom is shared with this atom. Likewise with four neighboring atoms and four neighboring atoms will share each valence electron with this atom. Therefore, total eight electrons are shared.
Covalent bonding in Germanium:
The outermost orbit of Ge has only four electrons. Ge atoms need four more electrons to become most stable. Ge atom forms four covalent bonds with the four neighboring atoms. In Covalent bonds each valance electron is shared by two atoms.
When Ge atoms come close to each other valence electron of atom is shared with neighboring atom and each valence electron of neighboring atom is shared with this atom. Likewise each atom will share four valence electrons with the four neighboring atoms and four neighboring atoms will share each valence.

The outermost shell of Si and Ge is completely filled and valence electrons are tightly bound to the nucleus of atom because of sharing electrons with neighboring atoms. In intrinsic semiconductors free electrons are not present at absolute zero temperature. Therefore, intrinsic semiconductor behaves as perfect insulator.

