The principle state that “no two electrons in the same atom can have identical values for all four of their quantum numbers. What this mean is that no more than two electrons can occupy the same orbital and those two electrons in the same orbital must have opposite spins. Because an electron spins, it creates a magnetic field, which can be oriented in one of two directions. For two electrons in the same orbital, the spins must be opposite to each other; the spins are said to be paired. These substances are not attracted to magnets and are to be diamagnetic. Atoms with more electrons that spin in one direction than another contain unpaired electrons. These substances are weakly attracted to magnets and are said to be paramagnetic.

Ψ= Ψ1 (a) Ψ2 (b) Ψ
Probability amplitude that electrons 1 are in state “a” and electron 2 is in state “b”.
Ψ1 Probability amplitude that electron 1 is in state “a”.
Ψ1 Probability amplitude that electron 2 is in state “b”.
The nature of the Pauli Exclusion Principle can be illustrated by supposing that electrons 1 and 2 are in the state a and b respectively. The wave function for the two electron system would be but this wave function is unacceptable because the electrons are identical and indistinguishable. To accounts for thaw we must use a linear combination of the two possibilities since the determination of which electron is in which state is not possible to determine.
Ψ= Ψ1 (a) Ψ2 (b) ± Ψ1 (b) Ψ2 (a)
Ψ Probability amplitude that both states “a” and “b” are occupied by electrons 1 and 2 in either order.
± Require for bosons and fermions
The wave function for the state in which both states “a” and “b” are occupied by electrons can be written. The Pauli Exclusion Principle is part of one of our most observations of nature: particles of half- integer’s spin must have anntisymmetric wave functions, and particles of integer spin must have symmetric wave function. The – sign vanish identically if both states are “a” and “b”, implying that it is impossible for both to occupy the same state.
Electronic configuration:-
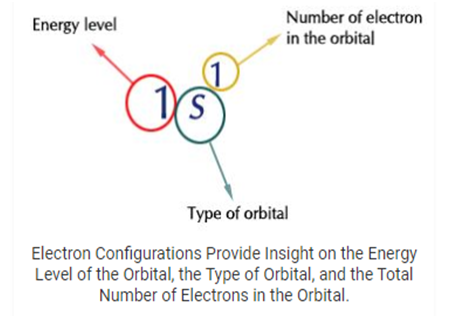
The electron configuration of an element describes how electrons are distributed in its atomic orbital’s. Electron configurations of atoms follow a standard notation in which all electrons- containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s² 2s²2p²3s¹.
However, the standard notation often yields lengthy electron configurations (especially for elements having a relatively large atomic number). In such cases, an abbreviated or condensed notation may be used instead of the standard notation.
In the abbreviated notation, the sequence of completely filled subshells that correspond to the electronic configuration of a noble gas is replaced with the symbol of that noble gas in square brackets.

Electronic configuration is useful for:
- Determining the valence of an element.
- Predicting the properties if a group of elements (elements with similar electron configuration tend to exhibit similar properties).
- Interpreting atomic spectra.
This notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presently by Ernest Rutherford and Niel Bohr in the year of 1913.
Writing of electronic configuration:
Shells
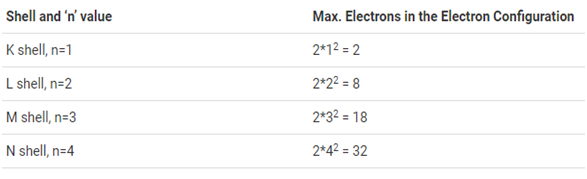
The maximum number of electrons that can be accommodated in a shell in based on the principal quantum number (n). It is represented by the formula 2n², where ‘n’ is the shell number. The shells, value of n, and the total number of electrons that can be accommodated are tabulated below.
Subshells
- The subshells into which electrons are distributed are based on the azimuthal quantum number (denoted by ‘ℓ’).
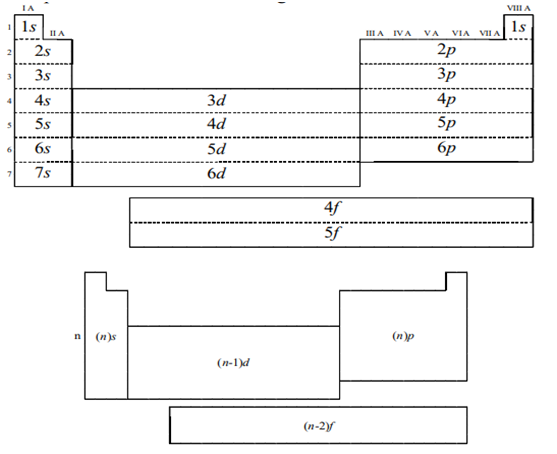
- This quantum number is depends on the value of the principle quantum number, n. Therefore, when n has a value of 4, four different subshells are possible.
- When n=4. The subshells corresponds ℓ=0, ℓ=1 and ℓ=3 and are named the s, p, d and f subshells respectively.
- The maximum number of electrons that can be accommodated by a subshell is given by formula 2*(2ℓ+1).
- Therefore, the s, p, d, and f subshells can accommodate a maximum of 2, 6 10, 14 electrons respectively.
All the possible subshells for values of n up to 4 tabulated below.
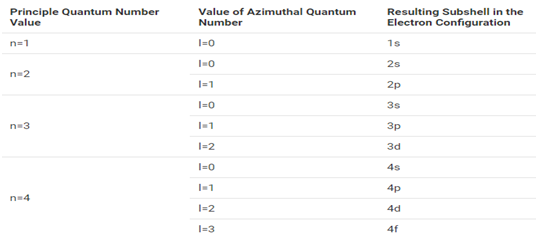
Thus, it can be understood that 1p, 2d and 3d orbital’s do not exist because the value of azimuthal quantum number is always less than that of the principal quantum number.
Notation:
- The electrons configuration of an atom is written with the help of subshell labels.
- These labels contain the shell number (given by the principal quantum number), the subshell name (given by the azimuthal quantum number), and the total number of electrons in the subshell in superscript.
- For example, if two electrons are filled in the‘s’ subshell of the first shell, the resulting notation is‘s’.
- With the help of these subshell labels, the electron configuration of magnesium (Atomic number 12) can be written as 1s² 2s² 2p6 3s².
Filling of Atomic orbitals:
Aufbau Principle: The Aufbau principle simply put, means electrons are added orbital’s as protons are added to an atom, the terms come from the German word “aufbau”, which means “built up” or “construction”. Lower electron orbital’s full before higher orbital’s do. “Building up” the electrons shell the electron shell. The end result is that the atom, ion, molecule forms the most stable electron configuration.
- The Aufbau principle dictates that electrons will occupy the orbital’s having lower energies occupying higher energy orbitals.
- The energy of an orbital’s is calculated by the sum of the principal and the azimuthal quantum numbers.
- According to this principle, electrons are filled in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p…
- An orbital’s can hold at most 2 electrons obeying the Pauli Exclusion Principle.
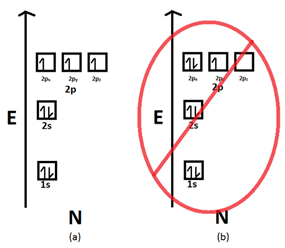
- Electrons obey Hund’s rule, which states that electrons spread out before that pair up if there are two or more energetically equivalent (e.g., p, d).
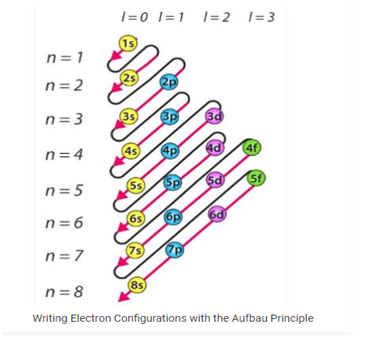
It is important to note that there exist many exceptions to the Aufbau principle such as chromium and copper. These exceptions can sometime be explained by the stability provided by half-filled or completely filled subshells.
A related term is the “Aufbau Rule” which states that the filling of different electron subshells is by order of increasing energy following the (n+1) rule.
Hund’s Rule: Aufbau principle tells us that lowest energy orbital’s get filled by electrons first. After the lower energy orbitals are filled, the electrons move on to higher energy orbitals. The problem with this rule is that it does not tell about the three 2p orbitals and the order that they will be filled in. According to Hund’s rule:
- Before the double occupation of any orbital, every orbital in the sub level is singly occupied.
- For the maximum of total spin, all electrons in single occupancy orbitals have the same spin.
An electron will not pair with another electron in a half-filled orbital as it has the ability to fill all its orbital’s with similar energy. A large number of unpaired electrons are present in atoms which at the ground state. If twp electrons come in contact they would show the some behavior as two magnets do. The electrons first try to get as far away from each other as possible before they have to pair up.
Its states that:
1: In a sublevel, each orbital is singly occupied before it is doubly occupied.
2: The electrons present in singly occupied orbitals possess identical spin.
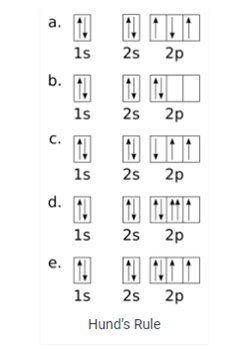
The electrons enter an empty orbital before pairing up. The electrons repel each other as they are negatively charged. The electrons do not share orbital’s to reduce repulsion.
When we consider the second rule, the spins of unpaired electrons is singly occupied orbital’s is the same. The initial electrons spin in the sub level decides what the spin of the other electrons would be. For instance, a carbon atoms electron configuration would be 1s² 2s² 2p². The same orbital will be occupied by the two 2s electrons although different orbitals will be occupied by the two 2p electrons in reference to Hund’s rule.
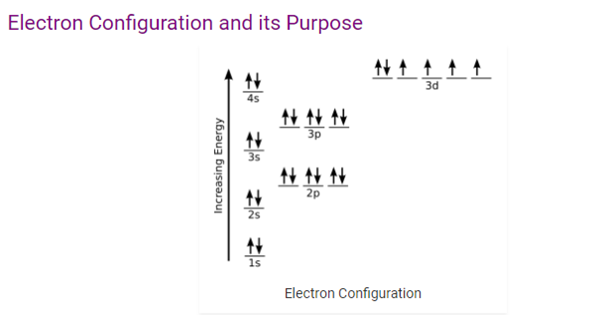
The above image helps in understanding the electronic configuration and its purpose. The valence shells of two atoms that come in contact with each other will be interact first. When valence shells are not full then the atoms is stable. The chemicals characteristics of an element are largely depend on the valence electrons. Similar chemical characteristics can be seen in elements that have similar valence numbers.
The stability can also be predicted by the electron configuration. When all the orbitals of atoms are full it is most stable. The orbital’s that have full energy level are most stable, for example, noble gases. These types of elements do not react with other elements.
The rule states that, for a stated electron configuration, the greatest value of spin multiplicity has the lowest energy term. It says if two or more than two orbital’s having the same amount of energy are unoccupied then the electrons will start occupying them individually before they fill them in pairs. It is a rule which depends on the observation of atomic spectra that is helpful in predicting the ground state of a molecule or an atom with one or more than one open electronic shells. This rule was discovered in the year 1925 by Friedrich Hund.
It is majorly used in atomic chemistry, quantum chemistry, and spectroscopy,

So, for example, if you are placing 4 electrons into the 2p orbitals, they would fill in the following order due to Hund’s rule: